[ad_1]
A late-stage trial of an Alzheimer’s drug set to generate outcomes inside weeks is shaping up as a pivotal second in a three-decade quest to show that eradicating sticky amyloid plaques from the mind can decelerate the illness.
The section 3 trial is being led by Eisai, a Tokyo-based pharmaceutical firm that has partnered US biotech Biogen to develop lecanemab. Earlier research recommended the monoclonal antibody therapy can clear plaques often called beta amyloid which might be on the centre of an more and more acrimonious scientific debate over what causes Alzheimer’s.
A constructive consequence might result in approval of a brand new drugs for a illness affecting 50mn victims worldwide that has no identified remedy. It could be encouraging for Eli Lilly and Roche, that are conducting trials on comparable medicine that would generate tens of billions of {dollars} in gross sales if they’re confirmed to sluggish the development of Alzheimer’s.
However scientists say disappointing check outcomes would deal a big blow to the so-called amyloid speculation, the concept that clearing clumps of poisonous cells that bind collectively within the mind can sluggish the speed of cognitive decline in victims.
Alberto Espay, professor of neurology on the College of Cincinnati, stated some researchers had develop into far too tethered to the amyloid speculation, which has been examined in dozens of research which have failed to supply conclusive proof that eradicating the plaques slows cognitive decline.
“We’ve run right into a dogma,” he stated. “And it is rather arduous to check new concepts when the overarching theme of funding is centred on the concept that eradicating amyloid should be the one approach to go.”
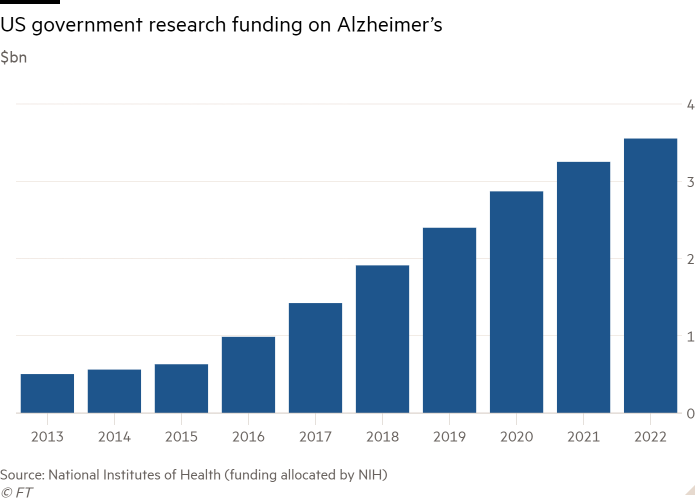
Disappointing outcomes may also act as a catalyst for a shift in funding for Alzheimer’s analysis, with some scientists arguing that promising areas of research and potential therapies have been crowded out by Massive Pharma’s give attention to amyloid.
The amyloid speculation is essentially the most examined of the various theories of what causes Alzheimer’s, which vary from an irritation of some kinds of mind cells to the presence and formation of varied proteins within the mind. It has been the main focus of greater than a fifth of the greater than 2,000 medical trials associated to the illness as of 2019.
The botched launch final 12 months of Biogen’s aducanumab — the primary amyloid-clearing drug to win approval and the primary new therapy for the illness in nearly 20 years — has served solely to intensify doubts over comparable medicine.
Aducanumab, which is bought beneath the model title Aduhelm, was given the fast-track inexperienced gentle by US regulators regardless of questions over its effectiveness and the robustness of two late-stage medical trials that underpinned its approval. Widespread scepticism amongst clinicians deepened additional when the corporate priced the therapy at $56,000 a 12 months, a transfer that additionally sparked a backlash amongst politicians and policymakers.
In April US authorities delivered a crippling blow to Aduhelm by severely limiting reimbursement by government-funded well being schemes, a transfer that limits its use to a couple thousand folks participating in medical trials. Any comparable amyloid therapies permitted beneath the FDA’s fast-track process would face the identical restrictions, a hurdle that Eisai acknowledges complicates the approval course of for lecanemab.
“Sure I admit that it does elevate the bar. That’s the reason the design of the trial . . . is so necessary,” Ivan Cheung, US chief government of Eisai, stated in an interview.
In a bid to construct public belief, Eisai is operating one of many largest trials ever undertaken on an Alzheimer’s drug, enrolling 1,795 sufferers within the early levels of the illness. It has additionally sidelined its companion Biogen by assuming what Cheung describes as “ultimate decision-making authority” because the drug strikes via the regulatory course of.
Eisai is aiming to match or higher the outcomes of an earlier trial that confirmed giving sufferers a 10mg dose of lecanemab each two weeks over 18 months can sluggish the speed of cognitive decline by 26 per cent, in contrast with those that got a placebo.
It’s utilizing the Scientific Dementia Ranking scale to measure the signs of dementia in sufferers in six classes, together with reminiscence, judgment and drawback fixing.
Critics allege this scale is an imprecise mechanism and query whether or not it’s value approving amyloid medicine which will solely barely scale back the tempo of cognitive decline and that may trigger life-threatening negative effects.
However affected person teams such because the Alzheimer’s Affiliation say even comparatively small delays in illness development can present vital advantages to folks struggling a terminal illness.
“It might imply six extra months in that stage the place you’ll be able to preserve your independence, take pleasure in your loved ones and attend a marriage,” stated Maria Carrillo, Alzheimer’s Affiliation chief science officer.
Regardless of the controversy over Aduhelm, Carrillo stated it was an encouraging time for Alzheimer’s analysis, pointing to elevated authorities funding and key findings from medical trials.
Eisai stated that following the Aduhelm controversy a consequence exhibiting a price of slowing beneath 25 per cent would possibly “bother” its FDA utility for accelerated approval for lecanemab, a course of that is because of conclude in January.
Beneath this fast-track course of, the FDA might approve the drug on the premise that it reduces amyloid plaque and is simply “fairly seemingly” to foretell a medical profit. These have been the identical standards used to approve Aduhelm, a contentious choice that prompted the resignation of three members of a committee advising the FDA on the drug.
Cheung stated he was assured the lecanemab trial can be successful and requested that amyloid sceptics research the information earlier than passing judgment. “All the things needs to be reality based mostly . . . I hope we may have a good debate,” he stated.
For Biogen, success would possibly assist rebuild its tarnished popularity following the disastrous launch of Aduhelm, which sparked $1bn of cost-cutting, the departure of its chief government and investigations by a number of US authorities businesses.
Ronald Petersen, director of the Mayo Clinic Alzheimer’s Illness Analysis Heart, stated if the lecanemab trial was a “flat-out adverse” then it might not be good for the “amyloid speculation”. However he stated it might not bury the idea altogether due to forthcoming outcomes from late-stage trials of three medicine from Roche and Eli Lilly.
Petersen stated his greatest guess was that one of many trials would present a constructive medical affect, though of a modest magnitude.
“This is able to give us a foot within the door for therapies as a result of finally down the highway its going to take mixture remedy to have a voilà type of impact,” he stated.
“If all 4 of those [trials] actually present no proof of any type of a medical affect . . . it could simply recommend we most likely ought to look elsewhere for medical targets,” he added.
Source link
